Introduction: The End of the Antibiotic Era?
Since their discovery, antibiotics have saved millions of lives and transformed modern medicine. Yet, we are now entering a critical crossroads: antimicrobial resistance (AMR) is rising at an alarming rate.
The World Health Organization (WHO) warns that by 2050, drug-resistant infections could kill more people each year than cancer.
If antibiotics lose their effectiveness, even routine surgeries and minor injuries could once again become life-threatening. This looming crisis has ignited an urgent search for alternatives — natural, microbial, and technological — that might help us outsmart resistant pathogens.
But a key question remains: Are these new therapies ready for everyday use?
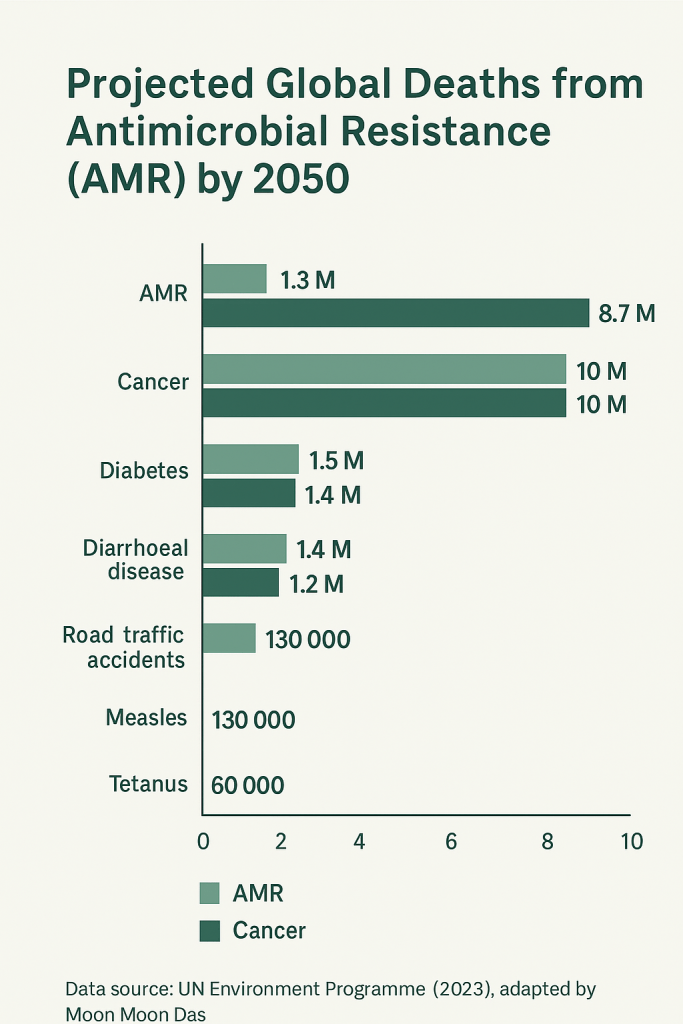
Why We Need Alternatives to Antibiotics
Antibiotics are losing their edge because of three interlinked factors:
- Overuse: Excessive use in humans, livestock, and agriculture accelerates resistance.
- Spread of Superbugs: Strains like MRSA and CRE are spreading globally.
- Declining Drug Development: Few new antibiotics are reaching the market, as pharmaceutical incentives dwindle.
Without new solutions, we risk slipping back into a pre-antibiotic age.
Nature’s Hidden Arsenal: Emerging Alternatives
Scientists are developing a diverse set of strategies to supplement or even replace antibiotics. Each offers a glimpse into a possible post-antibiotic future.
1. Phage Therapy
Bacteriophages — viruses that infect bacteria — can precisely target and destroy pathogens.
- Advantage: Highly specific; they spare beneficial microbes.
- Example: In 2019, a UK teenager was rescued from a deadly Mycobacterium infection using engineered phages.
- Status: In clinical trials, with some compassionate-use success stories.
2. Antimicrobial Peptides (AMPs)
Small proteins such as defensins and frog-skin peptides that puncture bacterial membranes.
- Advantage: Broad-spectrum activity against multiple pathogens.
- Challenge: Expensive to produce and prone to degradation inside the body.
3. CRISPR-Based Antimicrobials
This gene-editing technology can disable resistance genes or selectively kill harmful bacteria.
- Promise: Precision targeting with minimal collateral damage to healthy microbes.
- Status: Still in preclinical research but holds immense potential for precision medicine.
4. Microbiome-Based Therapies
Harnessing “good” bacteria to outcompete pathogens.
- Example: Fecal microbiota transplantation (FMT) already treats recurrent C. difficile infections.
- Future Potential: Designer probiotics and live biotherapeutics may one day prevent or treat a range of infections.
5. Nanotechnology
Metallic and polymer-based nanoparticles can penetrate bacterial biofilms — protective layers that block many antibiotics.
- Application: Used in wound dressings and antimicrobial coatings for medical surfaces.
6. Immunotherapies and Vaccines
Rather than attacking bacteria directly, these approaches boost the body’s immune defense.
- Example: New vaccines are being developed to target resistant bacterial strains.
Together, these innovations form the foundation of a post-antibiotic arsenal that may transform how we treat infection.
From Lab to Pharmacy: How Accessible Are These Options?
Scientific breakthroughs mean little without accessibility. Here’s how close some alternatives are to everyday use:
| Therapy | Availability |
|---|---|
| Probiotics | Readily available in stores, mainly for wellness support rather than infection treatment. |
| Silver-based nanoparticle dressings | Used in hospitals, especially for burn care. |
| Phage therapy | Offered in specialized clinics (e.g., Georgia and Poland) under limited conditions. |
| Fecal microbiota transplant (FMT) | FDA-approved for certain gut infections in the U.S. |
While you can’t yet buy a “phage pill” at your local pharmacy, the transition has begun.
The Road Ahead: What the Future Might Look Like
Experts envision a blended future — not a world without antibiotics, but one where smarter, targeted, and sustainable therapies take center stage.
- Precision Therapy: Custom phages or probiotics tailored to individual infections.
- Preventive Medicine: Microbiome-based strategies and vaccines reduce antibiotic reliance.
- At-Home Kits: Rapid infection tests linked to targeted treatments, much like COVID-19 test kits.
- Combination Therapies: Nanoparticles, peptides, and low-dose antibiotics working together to outsmart resistance.
The Challenges
- Cost: Advanced therapies may initially be expensive, raising equity concerns.
- Regulation: Agencies struggle to classify living or hybrid biological drugs.
- Access: Ensuring these innovations reach lower-income regions is vital.
- Public Awareness: Many people remain unaware of antibiotic alternatives or the importance of using antibiotics responsibly.
Is It Realistically Possible for Common People?
Short-term (Now – 5 years):
Limited access to alternatives like FMT, probiotics, and silver-based wound care. Phage therapy remains experimental.
Medium-term (5 – 15 years):
Phage therapy and microbiome-based drugs enter wider clinical use. Hospitals adopt personalized infection testing.
Long-term (15+ years):
Pharmacies may offer “precision antimicrobials” crafted from personal microbiome profiles.
Antibiotics persist, but as a backup tool — not the frontline defense.
So yes — life after antibiotics is possible, but it will unfold gradually. Affordability and equitable access will determine how inclusive that future becomes.
Scope for Future Work
Future research should focus on scaling up production, standardizing regulatory frameworks, and evaluating long-term ecological effects of microbiome manipulation. Global policy must align innovation with public health goals to ensure that these advances benefit everyone — not just a privileged few.
Takeaway: Living Smarter with Microbes
We are not yet living in a fully post-antibiotic world, but we are building the foundation for one.
For now, the most effective actions remain simple:
- Use antibiotics responsibly.
- Support your microbiome through diet, hygiene, and lifestyle.
- Stay informed about new therapies as they move from labs to clinics.
The future will not erase antibiotics — it will redefine our relationship with microbes.
Our goal isn’t to win a war against them but to coexist more intelligently.
What steps will you take today to help preserve tomorrow’s cures?